Next-Generation T7 Polymerase

optimized for purity, yield, and performance in modern IVT workflows:
- a direct replacement for WT T7 that delivers higher mRNA yields and greater mRNA integrity, particularly for challenging mRNA sequences such as sa-mRNA.
- can be used with chemically-modified LineaDNA™ IVT templates and a proprietary buffer to drastically reduce dsRNA contamination.
LineaRNAP™ Polymerase sets a new standard for in vitro transcription (IVT) workflows, offering researchers and manufacturers a powerful alternative to wild-type T7 RNA polymerase. Engineered with a proprietary DNA-binding domain, LineaRNAP delivers the high-fidelity and high-yield transcription expected of T7 while minimizing the production of immunogenic double-stranded RNA (dsRNA) byproducts – a common challenge in mRNA synthesis. This targeted activity simplifies downstream purification, increases mRNA recovery, and enhances the overall quality of the final product. Compatible with a range of co-transcriptional cap analogs and functional in high-salt conditions when paired with its proprietary buffer, LineaRNAP provides a cleaner, more efficient path to high-performance mRNA – making it a smart choice for developers focused on precision, purity, and yield.
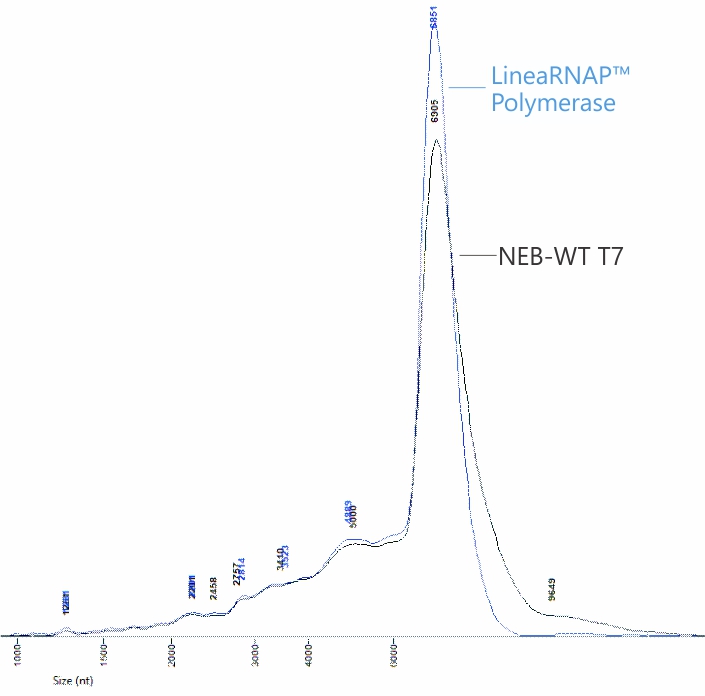
Analysis of mRNA products using RNA-seq and NGS revealed no difference in transcription fidelity between Linea RNAP and the wild-type enzyme. In addition, robust reductions in dsRNA and off target immune stimulation have been observed with mRNA produced using LineaRNAP. Also, LineaRNAP has been shown to produce higher mRNA yields with greater mRNA integrity versus WT T7, particularly for challenging mRNA sequences such as sa-mRNA.
Performance
High-Fidelity RNA Production
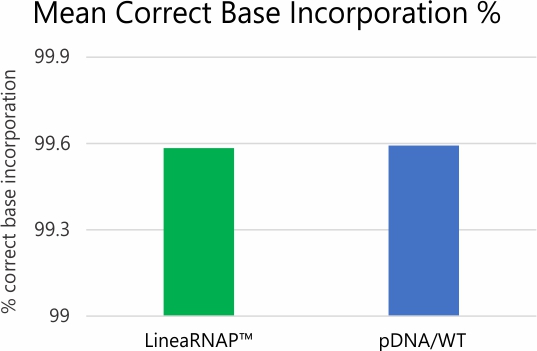
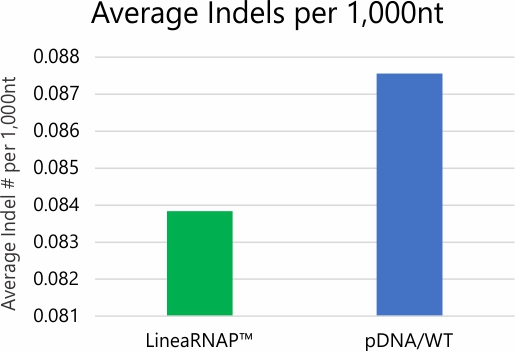
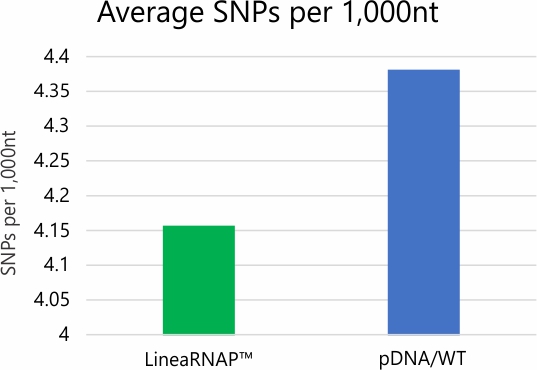
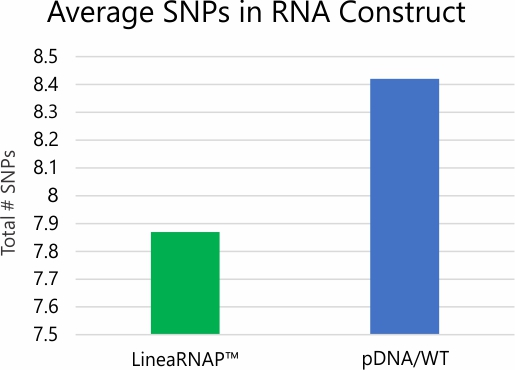
*Error rate and SNP/Indel analysis studies performed via deep read NGS
Greater mRNA Yields and Integrity
Lower dsRNA Content
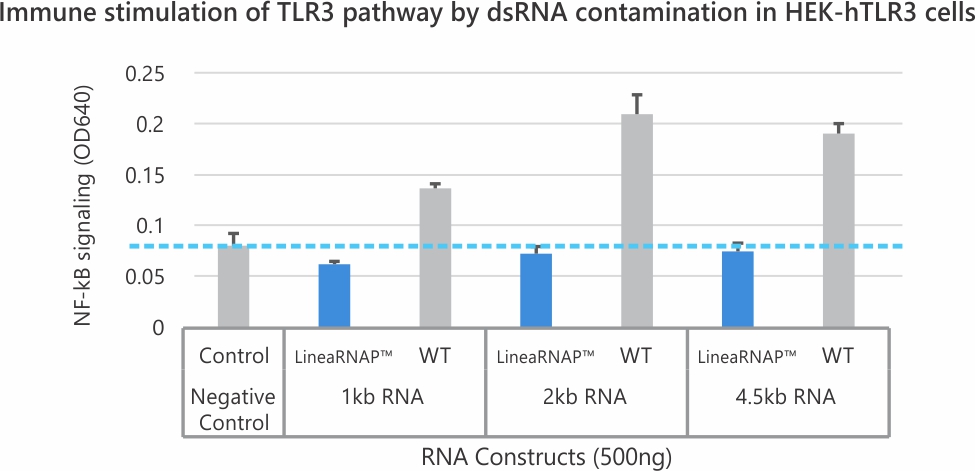
LineaRNAP™ streamlines mRNA production by reducing dsRNA impurities, improving yield, and enabling high-fidelity transcription under optimized conditions, empowering cleaner, more reliable IVT workflows.
GMP Excellence: Our processes and analytical methods are based on ISO9001/13485 quality systems ensuring that every product – whether destined for research or clinical application – meets rigorous quality requirements.