DNA Made Simple
DNA is not only the foundation of life. It’s also the foundation of modern medicine.
At LineaRx, an Applied DNA Sciences, Inc. (NASDAQ: APDN) company, we have a single purpose – to produce therapeutic DNA faster, of higher purity, and with greater scalability than current methods.
Therapeutic DNA is currently manufactured primarily via plasmids (pDNA), a complex biological method that has remained mostly unchanged for almost 40 years.
Our LinearDNA™ platform is a novel, enzymatic DNA manufacturing approach capable of producing high-quality DNA for nucleic acid-based therapies, including mRNA, Cellular and Gene Therapy and DNA Vaccines. We have named the resultant therapeutic DNA ‘LinearDNA’ or ‘linDNA’.
Our enzymatic approach and deep expertise in polymerase chain reaction (PCR) can dramatically accelerate innovation in nucleic acid-based therapies across a product lifecycle, from research and development to the clinic and commercial manufacturing.
The next generation of therapies require a next generation supply of DNA.
Linea™ IVT — Better mRNA…Faster
A Combination of 2 Next Generation Technologies
Linea IVT is the combination of LRx's enzymatically-produced Linea DNA IVT templates and its proprietary Linea RNA polymerase. The unique combination redefined mRNA production, resulting in:
A simplified mRNA workflow
Conventional IVT mRNA Production
Linea™ IVT mRNA Production
With significantly Reduced or Eliminated dsRNA Impurities
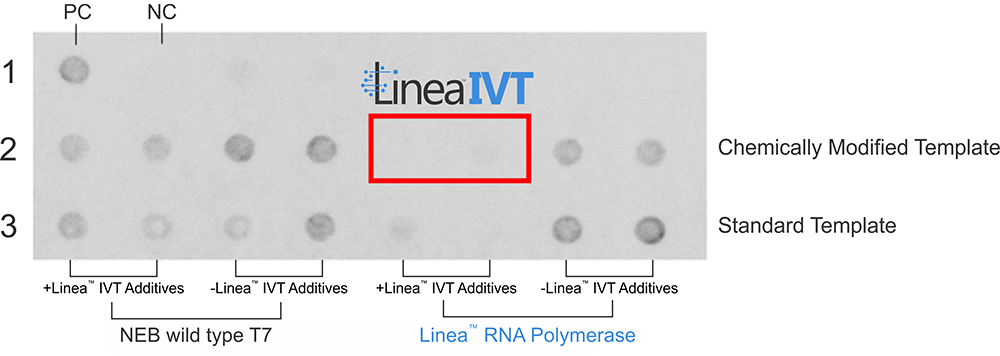
Without Sacrificing Yield
Enabling Nucleic Acid-Based Therapies
Our enzymatic LinearDNA platform enables us to make DNA at the Speed, Purity, Flexibility, and Scalability to support the rapid growth of nucleic acid-based therapies:
- Speed: days, not weeks or months
- Purity: 100% of produced DNA is comprised of the target therapeutic DNA sequence
- Flexibility: pDNA or a synthesis DNA template can be used
- Scalability: linDNA is available from the µg to g scale