a PCR-powered, cell-free breakthrough in DNA manufacturing delivering unmatched purity, precision, and scalability

LineaDNA, powered by a proprietary large-scale PCR platform, presents a breakthrough in DNA manufacturing, offering unmatched purity, precision, and scalability. Unlike other DNA production methods, which rely on bacterial cultures and extensive purification, LineaDNA is entirely cell-free, ensuring a higher-quality output with faster turnaround times. Its seamless scalability and ability to rapidly adapt to new technologies make it the ideal solution for meeting both current and future biotech industry demands.
The Future of DNA Manufacturing
While plasmid DNA has been widely used in the past, its reliance on bacterial growth creates purity, regulatory, and scalability challenges. RCA-based DNA production offers advantages for whole-genome amplification but introduces potential concatemer formation issues and still relies on plasmid DNA as a template. In contrast, PCR-based LineaDNA represents the most advanced and flexible DNA synthesis technology available today.
PCR – A Scalable Production Powerhouse
Our large-scale PCR platform is a powerful and reliable tool that delivers precision, versatility, speed, and cost-efficiency. It enables the amplification of a wide range of DNA sequences to create high-quality, sequence-defined DNA constructs essential for applications such as mRNA/IVT templates, DNA vaccines, gene editing, and personalized medicine. Rapid, reproducible, and scalable – PCR accelerates both research and production processes.
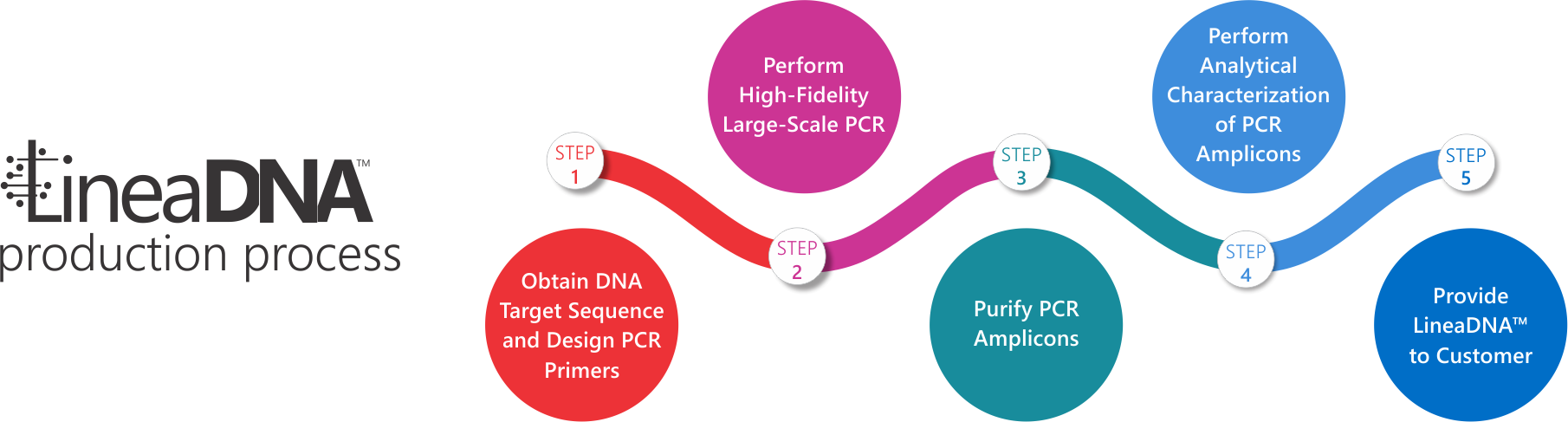
Advantages of LineaDNA Over Other Manufacturing Methods
LineaDNA offers a superior, streamlined process compared to plasmid DNA or rolling circle amplification (RCA)-based methods:
High Purity & Safety: 100% cell-free, eliminating bacterial contaminants, endotoxins, and antibiotic resistance genes commonly found in plasmid-based DNA.
Precision DNA Production: Produces only the target DNA, avoiding unwanted sequences seen in plasmid DNA (vector backbone contamination) or RCA (concatemer formation).
Faster, More Efficient Process: With only five production steps, LineaDNA can be manufactured in hours instead of days or weeks, enabling rapid large-scale production.
Scalability & Flexibility: LineaDNA can be produced from any double-stranded DNA source, including linear DNA and plasmid DNA, allowing for unmatched manufacturing adaptability.
Small Production Footprint: A compact, efficient process that enables high-yield DNA production without the need for large bacterial fermentation facilities.
At a Glance
| Feature | Plasmid-Based DNA | LineaDNA PCR-Based DNA | RCA-Based DNA |
| Amplification Method | Bacterial growth and extraction | Thermal cycling with DNA polymerase | Isothermal amplification with strand-displacing polymerase |
| Speed | Slow (days to weeks) | Rapid (hours) | Moderate (hours to day) |
| Scalability | Limited by bacterial culture size | Easily scalable with automation | Limited due to circular template requirements |
| DNA Structure | Circular | Linear | Mostly circular, often with concatemer artifacts. Can also be linear with closed ends. |
| Sequence Control | May contain unwanted bacterial sequences | Precise, with no extraneous elements | Possible concatemer formation |
| Purity | Requires endotoxin removal; may contain antibiotic resistance genes | High purity, no bacterial sequences | Potentially requires additional purification steps to remove circulate template DNA and processing enzymes |
| Contamination Risk | High risk (bacterial by products) and host cell contamination | Low (no bacterial involvement) | Low – Moderate |
| Downstream Applications | Gene therapy, biologics, research | IVT templates, gene therapy, synthetic biology | Whole genome amplification, diagnostics and biologics |
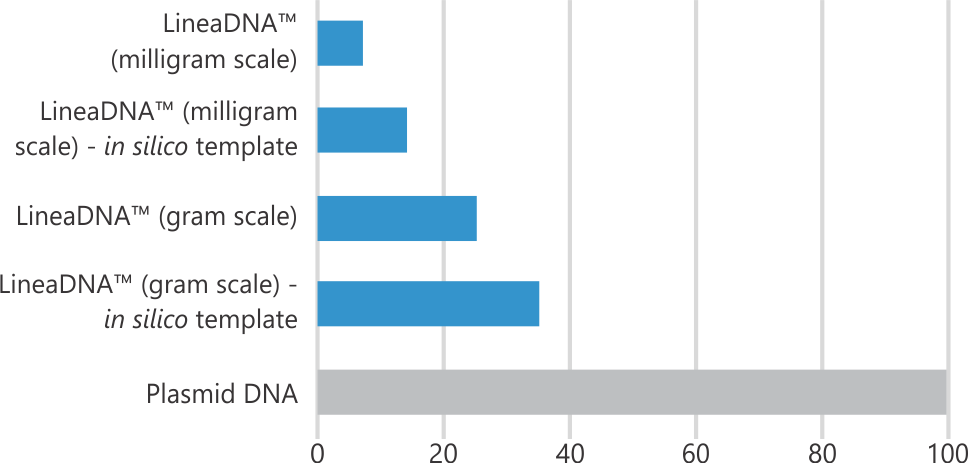
Performance
LineaDNA™ Manufacturing Timeline (days)
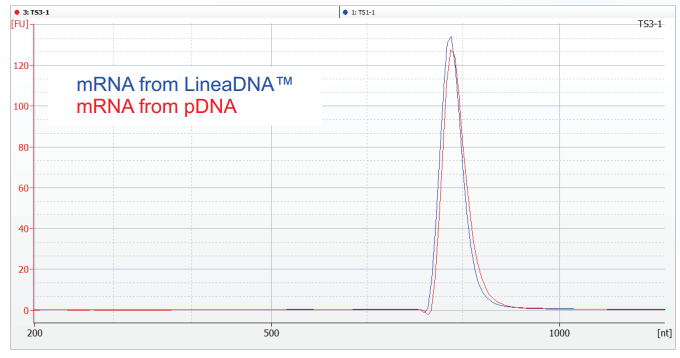
LineaDNA™ IVT Template Comparison to pDNA IVT Template
Applications
mRNA/IVT Templates:
efficient mRNA synthesis begins with robust, high-quality LineaRx production solutions

DNA Vaccines:
DNA vaccine development relies on the rapid generation of high-purity DNA.

Gene Editing:
high-precision DNA is paramount for successful gene editing applications.

Personalized Medicine:
in the era of personalized medicine, tailored therapies require the rapid production of custom DNA sequences.

GMP Excellence: Central to our approach is our state-of-the-art GMP production facility, which underpins all stages of production with rigorous quality and compliance standards. Our processes are based on ISO9001/13485 quality systems ensuring that every product – whether destined for research or clinical application – meets rigorous quality requirements.