PCR-based linear DNA templates optimized for in vitro transcription, enabling high-purity mRNA production for therapeutics and vaccine development.
LineaDNA™ IVT Templates from LineaRx are ready to use in vitro transcription (IVT) templates for mRNA production. Suitable for both R&D and clinical manufacturing our templates are produced using our proprietary LineaDNA technology, a polymerase chain reaction (PCR) based process designed to rapidly produce large quantities of high-quality IVT templates.

- Cell free, PCR-enabled enzymatic production process
- Rapid production timelines
- Eliminates the need for linearization and purification
- Primer induced modifications enable simple 3′ and 5′ IVT template customization
- Stable homopolymer amplification up to 180nt
- Various quality grades available, including GMP
- ISO9001/13485-based quality system
- Produced in New York based GMP facility
LineaDNA™ IVT Template Grades
| LineaRx | Research Grade | GMP-Like | GMP |
| Applications | Basic research, drug discovery, preclinical studies | Preclinical studies such as animal testing of drug safety and metabolism | Preclinical studies, clinical studies and commercialization |
| Production scales | 250 µg to 10 mg | 10 mg to gram scale | 10 mg to gram scale |
| Turnaround | 4 – 6 weeks (for new sequences) | <80 mg in 4 to 6 weeks > 80 mg to 500 mg 6 to 12 weeks > 500 mg 12 weeks + | <80 mg in 6 to 8 weeks > 80 mg to 500 mg 8 to 14 weeks > 500 mg 14 weeks + |
| Quality system | ISO9001 | ISO9001/13485 | ISO9001/13485 with applicable ICH Quality Guidelines |
| Production facility | Parallel production of orders in shared laboratory space | Production in dedicated space | Production in certified GMP suites |
| Document control and traceability | Yes | Yes | Yes, with additional systems and environmental controls |
| QC and release | Agilent Fragment Analyzer Nanodrop Agarose Gel HPLC (Purity) NGS Sequencing | Agilent Fragment Analyzer Nanodrop Agarose Gel HPLC (Purity) NGS Sequencing Endotoxin Sterility upon request | Agilent Fragment Analyzer Nanodrop Agarose Gel HPLC (Purity) NGS Sequencing Endotoxin Residual polymerase upon request |
| Aseptic fill/finish | Available upon request | Available upon request | Yes |
| Storage of retention sample | Available upon request | Available upon request | Yes |
| Document deliverable | DNA Analysis Report | 1. DNA Analysis Report 2. TSE/BSE statement upon request | 1. DNA Analysis Report 2. TSE/BSE statement upon request 3. Other documentation upon request |
LineaDNA™ IVT Template Comparison
to pDNA IVT Template
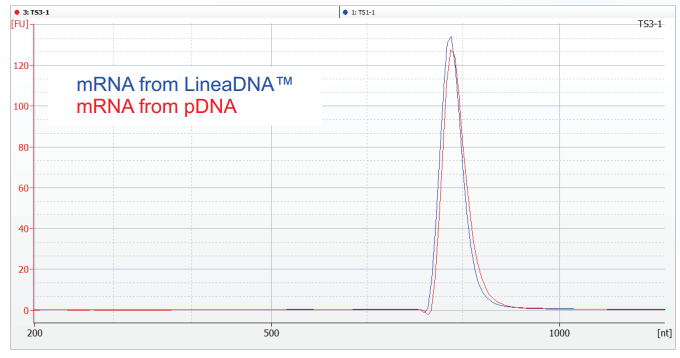

High Purity, Fidelity and Yields:
Our IVT templates are free from extraneous DNA sequences and are produced using a proprietary enzyme system that produces the highest possible sequence fidelity ensuring clean IVT reactions with maximum yields and minimal impurities.

Scalable and Rapid Manufacturing:
The PCR-based production process enables fast, high-yield DNA production suitable for both research and clinical applications. By eliminating the shortcomings of other manufacturing systems and providing a flexible platform for producing DNA at different grades – research, GMP-like, and GMP-PCR-enabled, enzymatic DNA synthesis stands out as leading-edge solution for today’s biotech and pharma industries.

PCR-Enabled Enzymatic IVT Template Production:
Unlike other DNA manufacturing processes, LineaDNA IVT templates are produced in a completely cell-free process, eliminating risks of endotoxin and other bacterial contamination, unwanted sequences and heterogenous homopolymer composition.

GMP Excellence:
Central to our approach is our state-of-the-art GMP production facility, which underpins all stages of production with rigorous quality and compliance standards. Our processes are based on ISO9001/13485 quality systems ensuring that every product -whether destined for research or clinical application – meets rigorous quality requirements.