AUTHORS
Atsuko Sangria, PhD, Director, Drug Substance Process Development, Kudo Biotechnology
Aaron Chung, Director, Nucleic Acid Platforms, LineaRx/Applied DNA Sciences
Yuhua Sun, Executive Director of R&D, LineaRx/Applied DNA Sciences
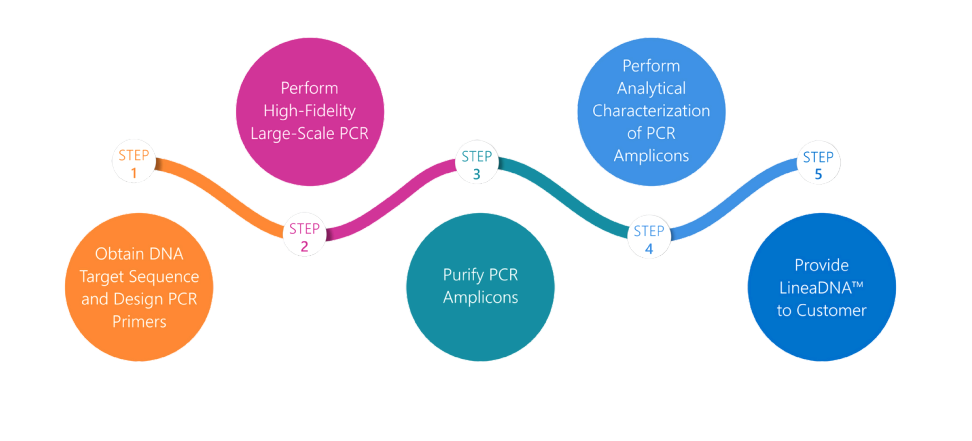
Overview
mRNA therapeutics offer benefits far beyond pandemic vaccines, including cancer vaccines, protein replacement, and cell and gene therapies. Swift development and delivery are crucial, as delays impact patient outcomes and commercial decisions by drug developers. Speedy production of cGMP grade high quality mRNA drug substance and drug product at an appropriate scale remains a challenge. One of the bottlenecks is the reliance on microbial fermentation to generate plasmid DNA (pDNA) which is used as the template for mRNA production, often slows market entry for new treatments.

