a next-generation in vitro transcription platform combining PCR-enabled LineaDNA™ templates and LineaRNAP™ polymerase to enhance mRNA production efficiency, streamline workflows, and minimize dsRNA contamination for faster, higher-quality mRNA synthesis.
LineaIVT™ is a next-generation in vitro transcription (IVT) platform designed to enhance mRNA production efficiency and quality. It features the combination of synthetic, chemically-modified LineaDNA™ IVT templates and LineaRNAP™, a novel highfidelity RNA polymerase linked to a DNA-binding domain. This advanced platform enables the production of higher-quality mRNA faster through simplification of workflows and significant reduction of double-stranded RNA (dsRNA) contamination.
- Significantly simplified mRNA production
- Reduced or eliminated dsRNA contamination
- Delivery of commercial scale, synthetic IVT templates in as little as 14 days
- Integrable into current mRNA workflows
Linea™ IVT mRNA Production (6 weeks or less*)
Conventional IVT mRNA Production 1 (6-8 months)
*based on internal Company data and modeling
timelines not shown to scale
LineaDNA™ IVT Templates
Synthetic LineaDNA IVT templates are designed for in vitro transcription (IVT) applications, particularly in mRNA therapeutics and vaccine development. These templates are produced using proprietary LineaDNA technology, a polymerase chain reaction (PCR)-based process used for the large-scale enzymatic production of DNA.
LineaRNAP™ Polymerase
When combined with LineaDNA IVT templates, LineaRNAP enables the production of mRNA more efficiently and with higher quality compared to conventional methods
Performance
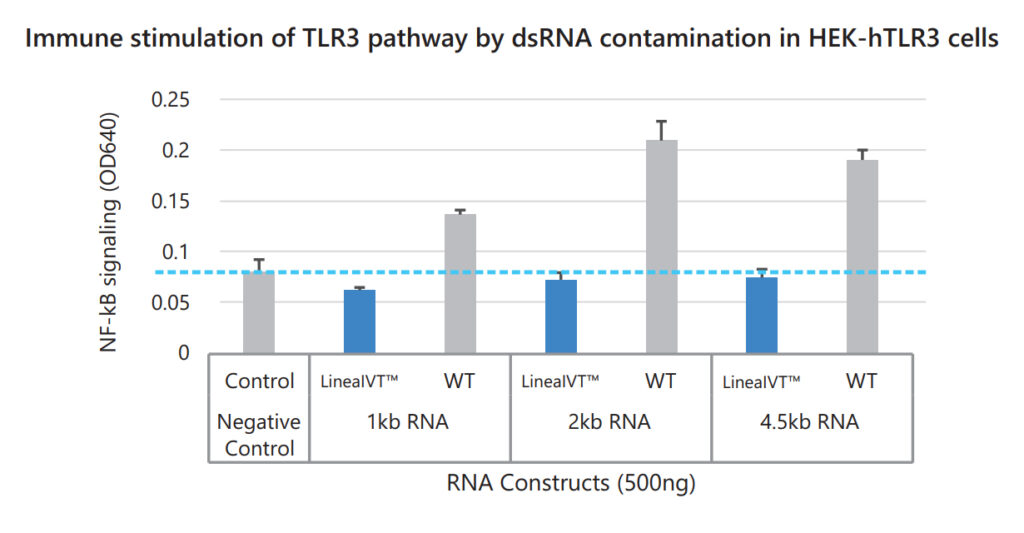
Lower dsRNA content
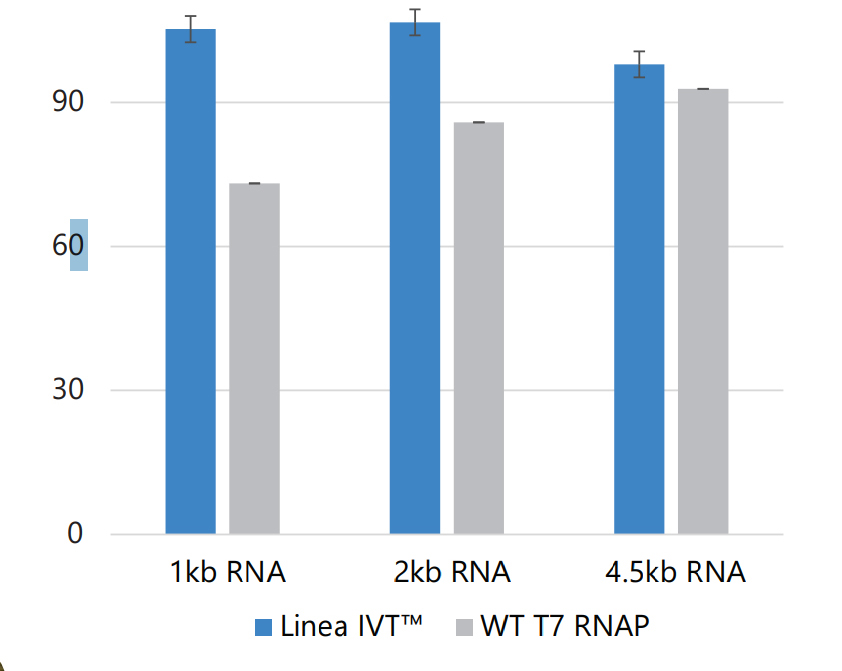
Greater RNA Yields
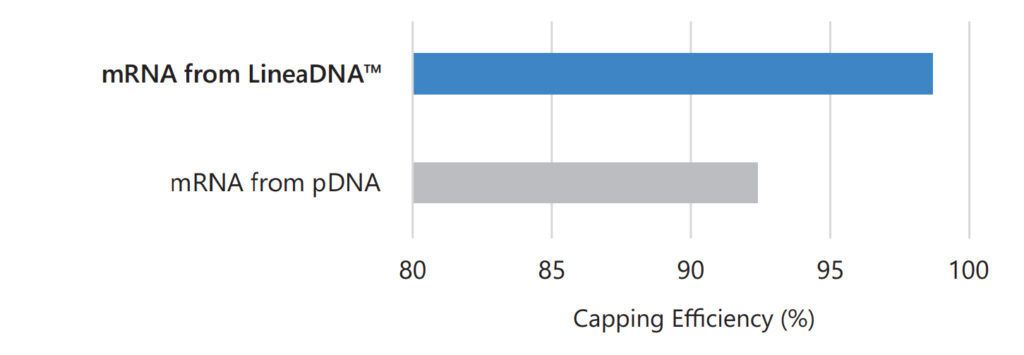
Improved Capping Efficiency
Applications
mRNA Therapeutics and Vaccines
Enable the production of custom mRNA for use in vaccines and therapeutic applications, including protein replacement and immunotherapies.
Cell and Gene Therapy
Used to generate mRNA for ex vivo cell engineering, such as CAR-T cell production or gene editing using systems like CRISPR.
Synthetic Biology
Facilitates rapid prototyping of genetic circuits and regulatory elements by enabling efficient in vitro transcription of design variants.
RNA-Based Diagnostics
Supports the synthesis of RNA standards, controls, and probes for molecular diagnostic assays, including RT-PCR and hybridization based methods.
Personalized Medicine
Enables fast-turnaround production of patient-specific mRNA constructs for personalized cancer vaccines or rare disease treatments.
GMP Excellence
Central to our approach is our state-of-the-art GMP production facility, which underpins all stages of production with rigorous quality and compliance standards. Our processes are based on ISO9001/13485 quality systems ensuring that every product - whether destined for research or clinical application - meets rigorous
quality requirements.