Efficient mRNA Synthesis Begins with Robust, High-Quality LineaRx Production Solutions
In the rapidly advancing field of mRNA therapeutics and vaccine development, the foundation of a successful in vitro transcription (IVT) process lies in the quality and integrity of the DNA template, RNA polymerase and buffer solutions to maximize efficiency, yield, and purity. The LineaRx production solutions of LineaIVT templates, LineaRNAP and buffers are delivered in quality grades for research, clinical, and commercial applications.

LineaIVT Templates: Customized for your sequence
Our LineaDNA platform leverages a proprietary PCR-based technology to deliver highly scalable, pure, and enzymatically-produced DNA IVT templates. This innovative approach provides a completely cell-free process, eliminating risks of endotoxin and other bacterial contamination, unwanted sequences and heterogenous homopolymer composition.
With LineaDNA, researchers and manufacturers benefit from:

High Purity and Homogeneity:
Free from unwanted bacterial sequences, LineaDNA is delivered with high consistency, ensuring reliable IVT performance.

Scalability and Speed:
The PCR-based production process enables rapid scaling without the limitations of traditional plasmid-based methods.

Ready-to-Use DNA:
LineaDNA is engineered to be immediately compatible with IVT reactions, eliminating the need for additional purification or modifications.

Optimized Poly-T Tails:
Homogeneous poly-T tails support efficient translation and reduce variability in mRNA synthesis.
Enhancing IVT Performance with LineaRNAP™ Polymerase
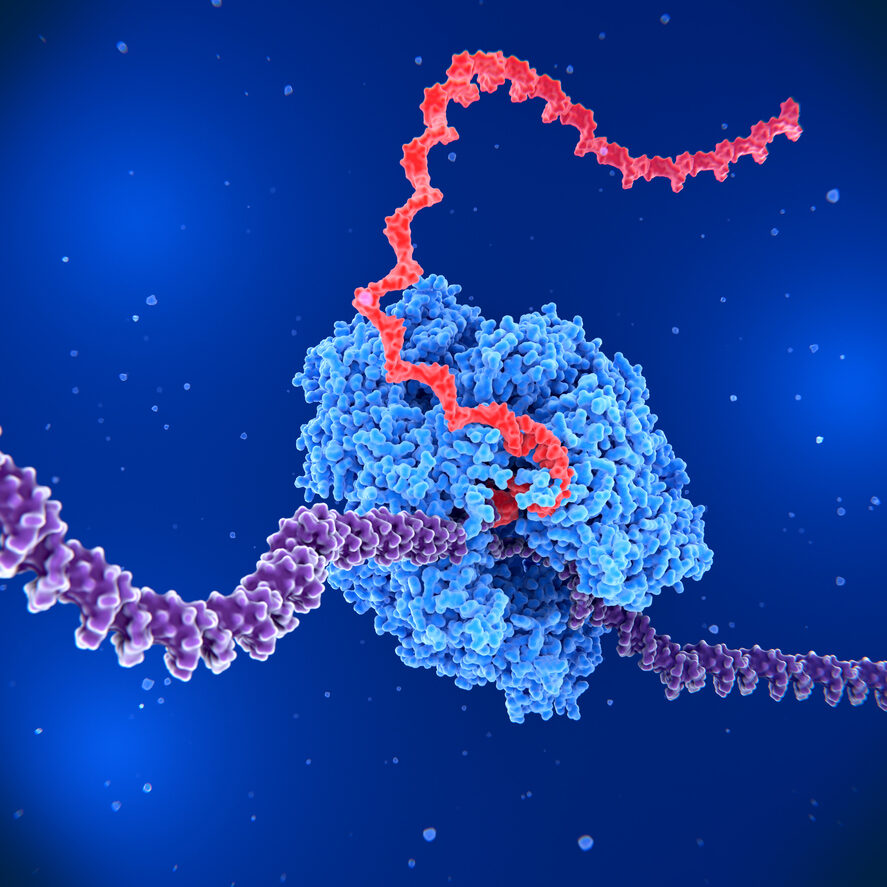

To further maximize the efficiency and quality of the IVT process, our LineaRNAP™ polymerase is designed to work synergistically with LineaDNA templates. This advanced polymerase formulation enhances transcription efficiency while minimizing the formation of double-stranded RNA (dsRNA) contaminants.
Key benefits of LineaRNAP™ polymerase include:

High Transcriptional Efficiency:
Maximizes mRNA yield while maintaining the integrity of the sequence.
Reduced dsRNA Impurities:
Lower levels of dsRNA prevent off-target innate immune responses, improving the safety and stability of mRNA therapeutics.

Enhanced Product Quality:
By reducing impurities, the resulting mRNA demonstrates improved performance in downstream applications, including vaccine and therapeutic production.
Efficient mRNA synthesis requires robust, high-quality DNA IVT templates that provide purity, scalability, and consistency. LineaIVT platform comprising DNA templates chemically-modified for tight integration with LineaRNAP polymerase delivers an optimized solution for mRNA production. LineaRNAP may also be used with 3rd-party DNA templates sourced as plasmid or other synthetic materials. Whether for research, preclinical studies, or commercial-scale manufacturing, our innovative approach sets a new standard for IVT template excellence, empowering scientists and manufacturers to produce high-performance mRNA efficiently and reliably.
