Powering nucleic acid-based therapies into the future with LinearDNATM
The global demand for DNA continues to rise at a breakneck pace. This trend began with the introduction of redirected cell and gene therapies and was supercharged by the success of mRNA COVID-19 vaccines. While these transformative therapies come in a variety of forms, they all have a common thread—they rely on DNA. According to a May 2021 report from the American Society of Gene and Cell Therapy, there were approximately 1,745 gene/cell therapies and 600 RNA therapies in development.1 These numbers have continued to grow over the past year. The net result is an unprecedented demand for DNA for research, clinical development, and therapeutic manufacturing.
Plasmid DNA—good enough, until now
To date, nucleic acid-based therapies have used plasmid DNA (pDNA) as their source of DNA. Based on 1970’s technology, pDNA was the only viable source of DNA when nucleic acid-based therapy development began and thus became the industry standard. pDNA has several limitations and downsides, including the presence of antibiotic resistance genes and origins of replication, inclusion of unwanted DNA sequences, low yields, batch failures and inconsistencies, as well as sequence heterogeneity—the latter two issues being exacerbated by the complex DNA sequences needed for gene and mRNA therapies. Moreover, pDNA manufacturing is complex and scaling its manufacture is both time-consuming and expensive. This fact has resulted in a pDNA supply that has failed to meet current DNA demands.
LinearDNA—breaking the DNA bottleneck
LinearDNA is an enzymatically produced DNA that is substitutable for pDNA in nucleic acid-based therapy manufacturing. LinearDNA is produced via the polymerase-chain-reaction (PCR) in a cell-free manufacturing workflow, and unlike plasmids, contains no extraneous DNA sequences. LinearDNA can be produced from an existing pDNA construct or a de novo DNA sequence.
LinearDNA is substitutable for pDNA in a wide range of nucleic acid-based therapy applications, including mRNA production, CAR-T, viral vector manufacturing and DNA vaccines. LinearDNA is produced via a rapidly scalable manufacturing platform that can grow to meet the needs of virtually any customer.
Gram-scale quantities of LinearDNA can be produced in mere weeks with high sequence fidelity and homogeneity—even with complex DNA sequences such as PolyA and inverted terminal repeats (ITRs). Recently, a proof-of-concept study was conducted in which a commercial scale batch of LinearDNA was produced from a de novo DNA template in 10 days—highlighting LinearDNA’s unparalleled speed of production.
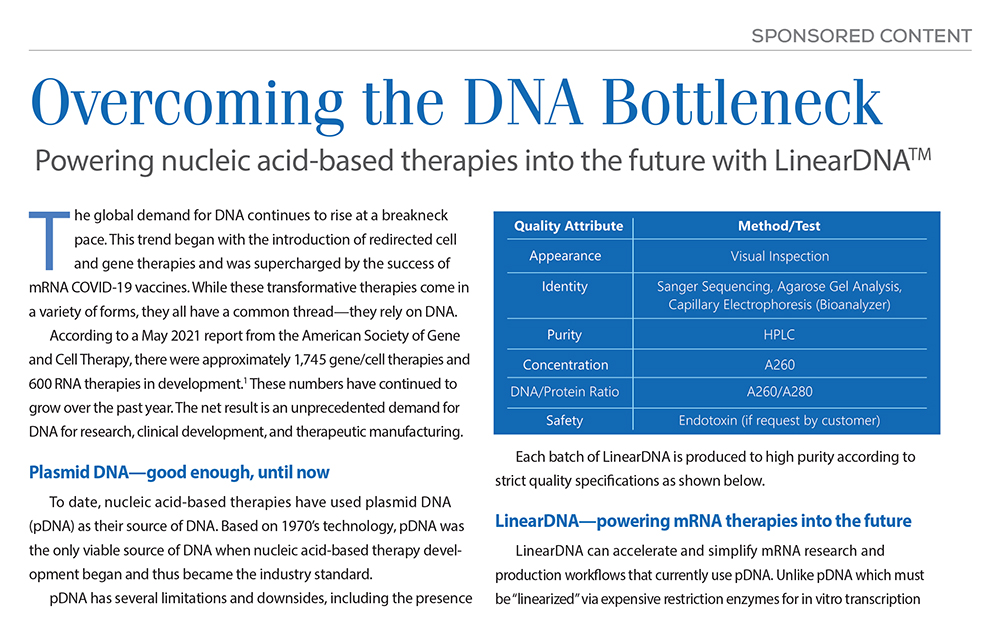
Each batch of LinearDNA is produced to high purity according to strict quality specifications as shown above.
LinearDNA—powering mRNA therapies into the future
LinearDNA is an enzymatically produced DNA that is substitutable for pDNA in nucleic acid-based therapy manufacturing. LinearDNA is produced via the polymerase-chain-reaction (PCR) in a cell-free manufacturing workflow, and unlike plasmids, contains no extraneous DNA sequences. LinearDNA can be produced from an existing pDNA construct or a de novo DNA sequence.
LinearDNA is substitutable for pDNA in a wide range of nucleic acid-based therapy applications, including mRNA production, CAR-T, viral vector manufacturing and DNA vaccines. LinearDNA is produced via a rapidly scalable manufacturing platform that can grow to meet the needs of virtually any customer.
Gram-scale quantities of LinearDNA can be produced in mere weeks with high sequence fidelity and homogeneity—even with complex DNA sequences such as PolyA and inverted terminal repeats (ITRs). Recently, a proof-of-concept study was conducted in which a commercial scale batch of LinearDNA was produced from a de novo DNA template in 10 days—highlighting LinearDNA’s unparalleled speed of production.
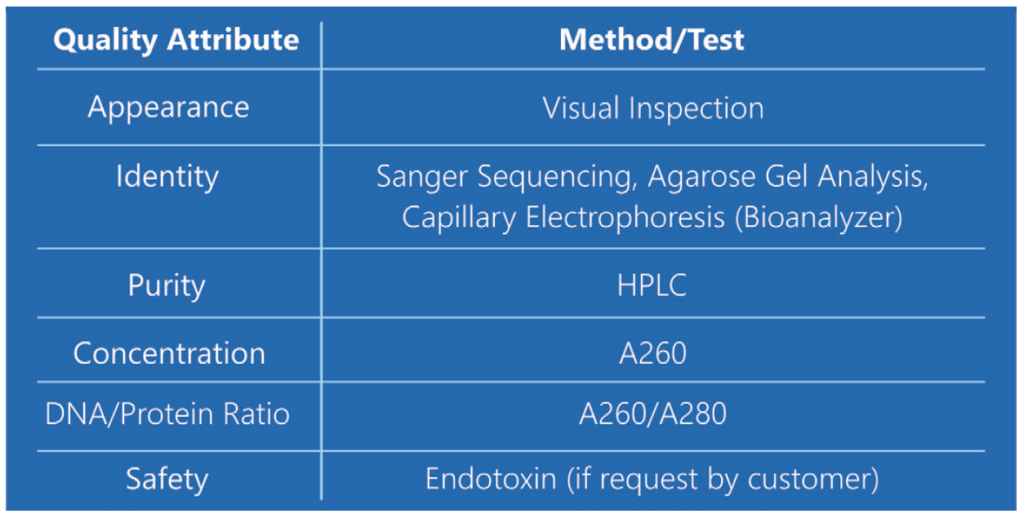
Each batch of LinearDNA is produced to high purity according to strict quality specifications as shown above.
Conclusion
pDNA, once the enabler of nucleic acid-based therapies, is now a problematic bottleneck. LinearDNA can rapidly meet all current and future DNA demands and remove DNA supply constraints on therapy developers and manufacturers. n
Reference
1. American Society of Gene + Cell Therapy Q2 2021 report